Client’s Testimonial:
“It has been a great experience working with you. The service and guidance you provided are worth every penny. I cannot thank you enough for your help.”
On April 16th, 2021, we received another EB-1A (Alien of Extraordinary Ability) approval for a Manager of Product Development in the Field of Pharmaceutical Sciences (Approval Notice).
General Field: Pharmaceutical Sciences
Position at the Time of Case Filing: Manager of Product Development
Country of Origin: India
State of Residence at the Time of Filing: Pennsylvania
Approval Notice Date: April 16th, 2021
Processing Time: 7 days (Premium Processing Requested)
Case Summary:
Holder of a Ph.D. in pharmaceutics and drug delivery, a manager of product development hired our law firm to file an EB-1A (Alien of Extraordinary Ability) petition representing his case. Before agreeing to countersign the retainer agreement, a thorough evaluation of his credentials was required to ensure the possibility of his EB-1A approval. We assessed his documents and established that he does possess an extraordinary ability to continue his research here in the United States. We validated it by elaborating on his qualification, current position, and research background with unique and expansive expertise to contribute significantly to the field of pharmaceutical sciences.
To reinforce our client’s EB-1A case, we detailed his intended research on new drug product development with a portfolio covering a range of therapeutic treatment areas. After a heedful review, we learned that his drug product and process development research is of the utmost importance due to their direct applicability in global efforts to address drug development and disease treatment, extending the fair expectation that he must stay to do so within the United States.
Additionally, we noted the 9 peer-reviewed scientific articles and 4 book chapters he published in international journals, which altogether amassed 308 citations. His findings have influenced many leading researchers in 7 countries, thereby demonstrating that his writings are eminent and widely relied upon in the field. Not only his citations and publications record led his current and future research in the field but the importance of his discoveries is also confirmed by the funding it has received from the National Institutes of Health (NIH), through two separate grants. This funding supports projects that promote the NIH in achieving its national healthcare goals and improving the quality of life in the U.S. Considering the inherent value of his drug delivery, drug product, and process development research, in succession with the government funding of his research, and the high esteem the scientific community holds for his work, it is clear that our client is one of the foremost scientists working in the pharmaceutical sciences field today.
Further, we highlighted that he has conducted 40 reviews for extraordinary and elite journals with very stiff publishing guidelines that enlist the services of only the most accomplished researchers. These statements certainly confirm his excellent work as a peer reviewer and are further evidence of his distinguished status.
Moreover, we incorporated 4 letters of recommendation gathered from fellow renowned experts in the field, confirming how our client’s work is fundamental for improving the therapeutic effect of drugs and public health and fighting illness in the United States. Two of them stated the following:
“Based on all of the above evidence, I hope it is clear that [client’s] expertise and accomplishments have firmly positioned him as one of the leading researchers in the pharmaceutical sciences community. Considering the novelty of his work and its important implications for real-world application, he has much to offer the United States.”
“[Client’s] work is crucial for the field for a multitude of reasons… The high costs, low success rate nature of drug discovery have positioned prodrug approaches such as his critical and cost-effective method for improving the absorption of protease inhibitor drugs, therefore is vital to domestic public health and economy.”
We are pleased that we were able to help our client in securing his EB1A petition approval within 7 days without any further request for evidence. A special thanks to Premium Processing service, a paid option offered by the USCIS, allowing petitioners to enjoy accelerated adjudication. We thank our client for choosing North America Immigration Law Group for representing his EB-1A application and for allowing us to be a part of his success story.
North America Immigration Law Group (Chen Immigration Law Associates) is a U.S. immigration law firm dedicated to representing corporations, research institutions, and individuals from all 50 U.S. states regarding I-140 immigration petitions. We specialize in employment-based immigration petition and have a proven record of high success rate for the categories of: EB2-NIW (National Interest Waiver), EB1-A (Alien of Extraordinary Ability), EB1-B (Outstanding Researcher/Professor) and O-1 (Alien of Extraordinary Ability).
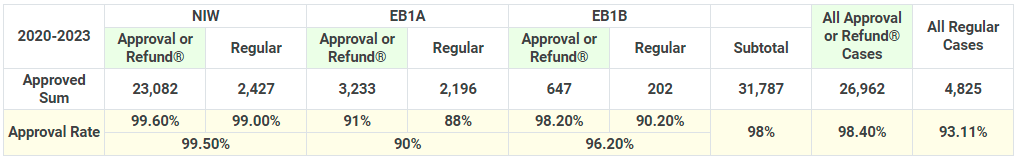
Our Ten Thousand I-140 Approvals Provide Unprecedented Insight into the USCIS Adjudication Trend
With more than 58,000 EB-1A, EB-1B, EB-2 NIW and O-1 cases approved, we have first hand information on the manner in which the USCIS adjudicate I-140 cases. As the USCIS has constantly changed its adjudication standards for the EB-1A, EB-1B and EB-2 NIW categories, our firm's huge database of successful cases gives you unprecedented insight to USCIS adjudication trends. We carefully analyze the data for all of our cases and apply the results of our analyses toward giving our clients up-to-date advice and adapting our strategies such that we remain on par with the ever-shifting landscape of immigration law in the U.S. With us, you will always have access to important updates, strategies, and information so that you can make the most informed decisions about your case.
We Have Helped Hundreds and Thousands of Clients with Credentials and Backgrounds Similar to Yours
With our exceedingly large number of successful petitions, no matter what credentials you have, no matter your background and field of expertise, no matter your visa status or nationality, chances are we have helped hundreds or even thousands of clients just like you. Our clients are usually impressed with how well we understand their research and work. Our insight and understanding stems from the fact that we have handled many cases with elements similar to yours already, and this helps us devise the best strategies for each individual petition.
Vast Majority of Clients Came to Us Because of Referrals
For years, our firm has attracted new clients based solely on word of mouth, recommendations, and the positive collaboration experiences shared with them by their friends and family. We take pride in our reputation and work hard to ensure that we provide a green card application experience that our clients are happy to share with their friends and colleagues. That is how our cumulative total of approved cases grew from 600 in 2013 to over 58,000 in 2025.
Approval Notices: https://www.wegreened.com/eb1_niw_approvals
Success Stories: https://www.wegreened.com/blog/
Website: www.wegreened.com
Free evaluation: https://www.wegreened.com/Free-Evaluation
Tel: 888.666.0969 (Toll Free)
To see more clients’ testimonials and approvals, please refer to:
To Learn More About Your Options CLICK HERE
Copyright © North America Immigration Law Group – WeGreened.com, All Rights Reserved.

